Herpes Virus Shows Promise in Shrinking Cancer Tumors
You could call U.S. Air Force veteran Russell Petrie’s arm a battlefield.
Since his melanoma diagnosis several years ago, the skin on his left arm has been cut, pulled and stitched. But the cancer kept coming back. This year, Petrie was given the option for a relatively new—and what some may call strange—treatment. His doctor wanted to inject him with a form of the herpes virus.

Dr. Jonathan Zager
"I had no qualms about it," said Petrie about telling his oncologist, Dr. Jonathan Zager, to move forward with the treatment. "I trust him wholeheartedly and he has taken care of me throughout all of this."
Zager was instrumental in bringing the treatment, called T-VEC, to the market, and was one of the principal investigators in the Phase 3 trial that led to FDA approval in 2015. He and others in Moffitt’s Cutaneous Oncology Program currently use the treatment frequently in dozens of patients per year.
T-VEC is a modified form of herpes simplex 1 virus that works by killing cancer cells in two ways: by directly causing cancer cells to burst and by activating the immune system to attack tumors. It is injected directly into the tumor, sometimes under ultrasound guidance, while the patient is awake.
Two of the genes in the virus have been removed so it can’t replicate in healthy cells, quashing any worries that a patient could contract herpes.
"In hundreds of cases I have never seen a herpetic lesion form or any herpes-type side effects,” said Zager. “There have also been no reports of a loved one contracting the virus."
Petrie comes to Moffitt every other week for his injection and in just two months his tumor has shrunk considerably to the point where it is nearly completely gone.
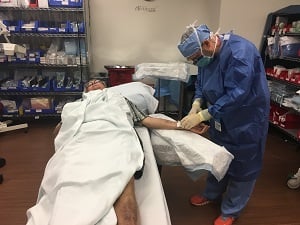
Russell Petrie receives a T-VEC injection as part of ongoing treatment for his melanoma.
"It was bigger than the nail on my thumb and sat off my wrist about a quarter of an inch high," said Petrie. "Now it’s probably half the size of my pinky fingernail and it’s flat."
While the herpes virus is only FDA approved for melanoma treatment, Moffitt’s Breast Oncology Program is conducting the first-ever T-VEC trial for the treatment of early stage triple negative breast cancer. Researchers are working to determine if injecting the virus directly into the tumor during chemotherapy and before surgery can help to eliminate triple negative breast cancer tumors.

Dr. Hatem Soliman
Results from the first phase of the study have shown the approach to be “safe and feasible,” said breast oncologist Dr. Hatem Soliman, who is leading the clinical trial. “We are seeing some impressive early results and hope to expand the use of T-VEC in the treatment of high-risk breast cancers in the near future.”
More viral-based studies will follow across other cancer types, Zager said. Combining virus-based vaccines with other immunotherapy treatments could be critical to improving patient outcomes, he said.