Melanoma Immunotherapy Offers Hope for Lung Cancer
An immunotherapy that has demonstrated durable responses in patients with melanoma is now showing promise for those with non-small cell lung cancer. Tumor infiltrating lymphocyte therapy, or “TIL,” uses a patient’s own live immune cells to fight cancer. Surgeons remove a patient’s tumor and, in the lab, dissect and culture the T cells inside. These cells, which were able to detect and invade the tumor, are then multiplied by the billions — a process that takes at least one month. Once infused back into the patient, the army of T cells can seek and kill the cancer cells.
“We know many of the immunotherapies that work well in melanoma can also benefit lung cancer patients. With that in mind, a group of investigators at Moffitt Cancer Center initiated a trial to determine if non-small cell lung cancer patients could have a similar response to TIL therapy,” said Dr. Ben Creelan, principal investigator of the phase 1 trial and associate member of the Department of Thoracic Oncology at Moffitt.
For the study, Moffitt recruited 20 patients with metastatic, non-small cell lung cancer. While their TIL therapy was being manufactured in the lab, they were treated with a different type of immunotherapy, a PD-1 inhibitor named Opdivo. Sixteen of the 20 patients saw tumor growth despite treatment with Opdivo. Those patients were then infused with personalized TIL therapy.

Dr. Ben Creelan with Sheri Pummill, a lung cancer patient who had a complete response on the TIL therapy trial.
“When we launched the study, we never expected to see complete responses. That is very rare in lung cancer. However, two patients did achieve a complete response, meaning no evidence of disease on their scans, from the personalized TIL therapy. Another one had a complete response with Opdivo alone,” said Creelan.
He said several other patients on the trial showed partial response to TIL therapy, making the overall response rate from the study at least 25%. Complete results from the phase 1 trial will be presented during the American Association for Cancer Research Annual Meeting.
“Another interesting thing to point out is that biomarkers, such as driver mutations, do not rule out a potential benefit with TIL therapy. We have patients with several types of cancer mutations on the trial, and their clinical benefit seems to be independent of mutation type,” noted Creelan.
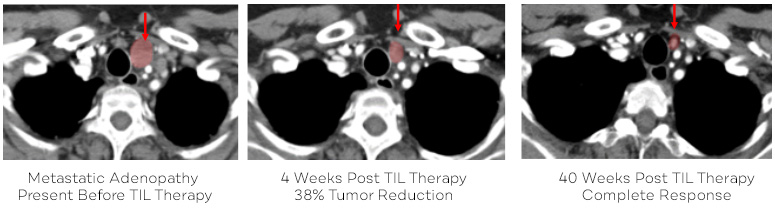
Sheri Pummill's scans showing her response to TIL therapy. This is one of several lesions that responded to the therapy.
Moffitt investigators are now looking at ways to make TIL therapy more accessible to more patients, both in terms of production time and tolerability. They are also researching how to enhance the TIL to increase the response rate. Moffitt is also opening Iovance Biotherapeutics’ clinical trial, which combines pembrolizumab with TIL for Stage 4 lung cancer.