Tumor-Infiltrating Lymphocyte (TIL) Therapy for Metastatic Melanoma

Each year, approximately 8,000 people in the United States die of melanoma, an aggressive skin cancer that begins in the pigment-producing cells (melanocytes) that give the skin its color. Metastatic melanoma—at its most advanced stage—occurs when the cancer spreads beyond its site of origin to a distant site in the body.
Traditionally, metastatic melanoma has been treated with a combination of surgery, chemotherapy, radiation therapy, immunotherapy and/or isolated limb perfusion and infusion (ILP/ILI). In some cases, however, the cancer does not respond to standard treatments. In others, the cancer recurs after initially responding to treatment (refractory metastatic melanoma).
TIL therapy: a breakthrough in metastatic melanoma treatment
A first-of-its-kind cellular immunotherapy pioneered at Moffitt Cancer Center is now an option for treating advanced melanoma. An intensive, “one-and-done” treatment, tumor-infiltrating lymphocyte (TIL) therapy is approved by the U.S. Food and Drug Administration (FDA) for treating solid tumors. The results of a recent landmark multinational study led by Moffitt showed that TIL therapy holds promise for treating refractory metastatic melanoma and that it is the most reliable option after traditional standard-of-care therapies have proven ineffective.
Specifically, in a revolutionary clinical trial, researchers evaluated the effects of Iovance Biotherapeutics’s lifileucel (AMTAGVI™)—a tumor-derived autologous T cell immunotherapy—on adults with advanced melanoma. The researchers found that lifileucel “demonstrated durable responses and addresses a major unmet need in patients with metastatic melanoma with limited treatment options after approved therapy, including in patients whose disease was primarily refractory to anti-PD-1.”
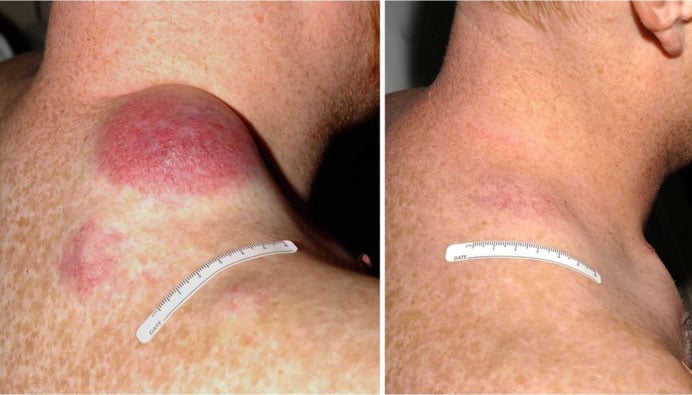
melanoma who underwent TIL treatment. Photo from National Cancer Institute
How does TIL therapy work?
TIL therapy capitalizes on the power of a patient’s own immune system to fight cancer. Found in blood and lymphatic tissues, T lymphocytes are immune cells that can recognize, target and destroy cancerous cells. T lymphocytes are considered to be TIL when they migrate to the tumor, but unfortunately, TIL are typically not able to mediate complete tumor destruction on their own.
TIL therapy involves surgically removing a portion of a melanoma tumor that contains the TILs that have invaded the tumor. The patient-specific TILs are then cultured and expanded (multiplied) in a laboratory using interleukin-2, a protein that promotes the growth and activity of other immune cells, such as TILs. Unlike CAR-T cell therapy, TIL therapy does not involve the genetic modification of immune cells; instead, it relies on the natural process of tumor rejection and amplifies it with a massive expansion in the laboratory.
Once a sufficient quantity of TILs is available, the patient receives chemotherapy to temporarily deplete their immune system and “clear space” for the new army of TILs, which are infused into the bloodstream. Once there, the TILs circulate throughout the body to actively seek out and eliminate cancer cells while leaving healthy cells unaffected.
Benefit from world-class care at Moffitt Cancer Center
An established leader in cellular immunotherapy, Moffitt is currently one of only three treatment centers in Florida authorized by Iovance Biotherapeutics to administer lifileucel (AMTAGVI™). We are continually building upon our successes, and our scientists and clinicians are already working on the next generation of TIL therapies for melanoma and other solid tumors, with several clinical trials underway.
Medically reviewed by Amod Sarnaik, MD, Cutaneous Oncology Program.
If you would like to know more about Moffitt Cancer Center or how TIL therapy is being used to treat metastatic melanoma, contact us today. You can reach us by calling 1-888-663-3488 or filling out our new patient registration form online (no referral is needed). Once you do, we’ll connect you to a cancer expert as soon as possible.
References
ClinicalTrials.gov: Expanded Access Program of Lifileucel (LN-144) in Patients With Unresectable or Metastatic Melanoma
PubMed.gov: Lifileucel, a Tumor-Infiltrating Lymphocyte Therapy, in Metastatic Melanoma
Moffitt’s Groundbreaking Work in TIL Therapy
The National Cancer Institute began studying tumor-infiltrating lymphocyte (TIL) therapy in the 1980s. As a cutting-edge cancer research institute, Moffitt aspired to be at the forefront of this potentially groundbreaking immunotherapy. In 2009, Moffitt researchers visited the NCI to learn the protocol for creating TIL therapy, and Moffitt became the first cancer center outside of the NCI to offer TIL to melanoma patients. Since then, Moffitt’s experts have remained on the cutting edge, exploring a variety of ways this innovative cell therapy could be used to save more lives.
-
19
TIL trials have been opened at Moffitt since researchers learned the protocol in 2009
-
184
Patients have been accrued to Moffitt’s TIL trials, contributing to the advancement of the therapy

