Stomach Cancer Treatment

Stomach cancer, also known as gastric cancer, occurs when abnormal cells grow uncontrollably in the lining of the stomach, forming a tumor. While the exact cause is unclear, scientists have identified several risk factors, including a diet high in smoked or processed foods, chronic infection with Helicobacter pylori, smoking, obesity and a family history of gastric cancer.
Early signs of stomach cancer are often subtle, such as indigestion, heartburn and a feeling of fullness after eating only a small amount of food. As the tumor progresses, more pronounced symptoms may develop, such as unexplained weight loss, persistent nausea, vomiting, difficulty swallowing and blood in the stool. Due to the vague nature of the initial symptoms, many cases are diagnosed in an advanced stage. Early detection is key to achieving the best possible outcome and quality of life.
Stomach cancer is typically treated with a combination of therapies, which may include surgical removal of the tumor, chemotherapy, radiation therapy and/or targeted therapy. The optimal approach can vary depending on the type, size, location and stage of the tumor and the age and overall health of the patient.

Ranked a Top Cancer Hospital in Nation
Moffitt is ranked 15th in the nation and is the top-ranked cancer hospital in Florida and the Southeast in Newsweek’s “America’s Best Cancer Hospitals."
Surgery for stomach cancer
Surgery is the primary treatment for early-stage stomach cancer, particularly when the tumor is small and remains confined to the area where it originated. The goal is to remove the tumor and any affected tissues, helping to prevent the cancer from progressing and spreading. Often, surgery is combined with chemotherapy or radiation therapy for heightened effectiveness.
Surgical treatment for stomach cancer may involve:
Endoscopic mucosal resection for stomach cancer
Endoscopic mucosal resection (EMR) is a minimally invasive procedure that can be performed to remove abnormal tissue from the gastrointestinal (GI) tract. EMR may be considered for treating early-stage stomach cancer, particularly when there is a low risk of metastasis to the lymph nodes.
During the procedure, a gastroenterologist will insert an endoscope—a small tube equipped with a light and camera—through the mouth or anus and guide it into the GI tract. Guided by real-time images created with the endoscope, the gastroenterologist will use specialized surgical tools to remove the abnormal tissue and a slim margin of surrounding healthy tissue.
Endoscopic mucosal resection is advantageous because it offers the ability to treat certain stomach tumors without the need for major abdominal surgery. However, it is a complex procedure that requires specialized expertise. Therefore, seeking care at a high-volume cancer center is essential to ensure the best possible outcome and quality of life.
While EMR may offer a potential cure for some patients with early-stage stomach cancer, additional treatment—such as chemotherapy or radiation therapy—may be necessary if the margin is found to be cancerous or if the tumor is more advanced than initially thought.
What is the recovery process like after endoscopic mucosal resection for stomach cancer?
In general, EMR results in a faster recovery than traditional stomach cancer surgery, but the process still requires close attention and specific care. This may include:
- Immediate post-procedure care – After endoscopic mucosal resection, the patient will be monitored in a recovery area for a few hours to ensure there are no immediate complications. Due to the use of sedation during the procedure, the patient may feel groggy for a short period but should be able to go home the same day.
- Pain management – Some discomfort is normal after EMR, such as a sore throat (if the endoscope was inserted through the mouth) or mild abdominal cramping. The physician will provide specific recommendations based on the patient’s unique situation. Usually, over-the-counter pain relievers are sufficient to manage any pain.
- Activity and dietary restrictions – The patient will be advised to rest for the first 24 hours and avoid heavy physical activity for several days. They may also be instructed to refrain from eating solid foods for a short time and gradually transition to a normal diet as tolerated. It is important to follow the physician’s instructions to avoid irritating the treated area.
- Follow-up appointments – The patient will regularly see their physician, who will monitor their progress and monitor for signs of cancer recurrence. The physician may recommend additional testing, such as an endoscopy, to confirm that the treated area is healing properly and that no cancerous cells remain.
- Further treatment – If the margin is cancerous, the physician may suggest further treatment, such as chemotherapy or radiation therapy, to address the residual cancer.
Overall, recovery from EMR is generally quick, with most patients returning to their normal activities within a few days. That said, the specific timeline can vary depending on the extent of the procedure and the patient’s health.
-
26,400
new stomach cancer diagnoses in the U.S. per year
-
11,000
deaths attributed to stomach cancer in the U.S. per year
What are the risks and possible complications of endoscopic mucosal resection for stomach cancer?
While EMR is a generally safe, minimally invasive procedure, it does have some associated risks and potential complications. These include:
- Excessive bleeding – One of the most common complications of EMR is bleeding at the site of the tissue resection. In most cases, the bleeding is minor and can be controlled during the procedure. Rarely, a patient may experience delayed bleeding that requires additional treatment.
- Infection – As with any procedure that involves the GI tract, there is a risk of infection, which can develop at the excision site or other parts of the digestive system. Antibiotics may be prescribed to prevent or treat infection.
- Perforation – A tear in the wall of the gastrointestinal tract is a rare but serious complication of EMR. If a perforation occurs, it can lead to infection of the abdominal cavity (peritonitis), which may require emergency surgical repair.
- Stricture formation – In some cases, scarring at the surgical site can cause a narrowing or stricture in the gastrointestinal tract. This can lead to difficulty swallowing or digestive problems that require further treatment, such as dilation to widen the narrowed area.
- Incomplete resection – Although EMR aims to remove all cancerous cells, there is a slight chance that some abnormal tissue may remain, especially if the lesion is large or difficult to access. In such cases, further treatment may be considered to ensure complete tumor removal.
- Cancer recurrence – If the margin is cancerous, there is a possibility that the cancer could come back. Close follow-up care and monitoring are essential to detect any early recurrence signs. Additional treatments, such as chemotherapy or radiation therapy, may also be considered.
- Anesthesia risks – Because endoscopic mucosal resection is typically performed under sedation or general anesthesia, the procedure carries risks related to the anesthesia, especially in patients with an underlying health condition. These risks are generally low but should be discussed with the physician beforehand.
- Pain and discomfort – While any post-operative pain is typically mild and manageable, some patients may experience ongoing discomfort or sore throat, which should eventually resolve on its own.
Partial gastrectomy for stomach cancer
Partial gastrectomy is a surgical procedure that involves removing a portion of the stomach to treat cancer or benign tumors. When used to treat stomach cancer, the procedure is performed by a surgical oncologist, who will remove the cancerous portion of the stomach along with some nearby lymph nodes for microscopic examination by a pathologist. The surgeon may also remove a portion of the spleen, pancreas, small intestine or colon if there are visible signs of cancer. If the lower portion of the stomach is removed, the surgeon will connect the upper portion of the stomach directly to the small intestine.
What is the recovery process like after partial gastrectomy for stomach cancer?
Proper care and adherence to medical advice can support successful healing from partial gastrectomy, which can take up to several months. During that time, the patient can expect:
- A hospital stay – Most patients spend five to seven days in the hospital for monitoring and pain management. Early movement will be encouraged to help prevent complications.
- Dietary progression – The patient will begin with intravenous (IV) nutrition, progress to clear liquids and gradually reintroduce soft and solid foods in small, frequent meals.
- Pain and fatigue – The patient can manage any post-operative soreness with prescribed medications and should balance rest with light activities to rebuild strength.
- Activity restrictions – The patient should avoid heavy lifting and strenuous activities for six to eight weeks and then resume their daily tasks gradually.
- Nutritional supplements – Due to changes in nutrient absorption, the patient may require lifelong vitamin B12, iron and calcium supplementation.
- Gastrointestinal discomfort – Nausea and diarrhea can be usually managed by avoiding sugary foods, eating small meals and resting after eating.
- Weight fluctuations – Weight loss is common after partial gastrectomy. With the guidance of a licensed dietitian, the patient can maintain a healthy body weight by following a balanced diet.
- Follow-up care – The patient will have regular appointments with their physician, who will monitor the healing process, check for surgical complications and address nutritional needs.
What are the risks and possible complications of partial gastrectomy for stomach cancer?
Partial gastrectomy is generally considered safe. However, like any surgery, it carries certain risks and potential complications. Understanding these risks can help the patient make better-informed treatment decisions and recognize possible warning signs during their recovery.
Common risks and complications associated with partial gastrectomy include:
- Infection – An infection may develop at the incision site or inside the abdomen, which may require antibiotics or further treatment.
- Bleeding – Blood loss can occur during or after surgery, sometimes necessitating a transfusion.
- Leakage – Seepage at the connection between the stomach and small intestine may require additional surgery.
- Dumping syndrome – Rapid emptying of the stomach contents into the intestine can cause gastrointestinal symptoms, such as nausea, cramping and diarrhea.
- Delayed gastric emptying – Slow movement of food from the stomach to the intestines may cause nausea or bloating.
- Nutritional imbalances – Post-surgical deficiencies in vitamin B12, iron and calcium may require lifelong supplementation.
- Weight loss – Significant weight loss can occur due to reduced stomach capacity and dietary adjustments, which can be addressed with the help of a dietitian.
- Stricture formation – Scar tissue can cause narrowing in the digestive tract, leading to difficulty eating or swallowing.
- Blood clots – As with any surgery, there is a risk of deep vein thrombosis (DVT) or pulmonary embolism, which can be reduced with early mobility.
- Fatigue – Many patients feel weak and tired during recovery from partial gastrectomy, often due to dietary changes and reduced calorie intake.
Total gastrectomy for stomach cancer
Total gastrectomy is a surgical procedure that involves removing the entire stomach to treat advanced or widespread cancer. By removing the stomach and reconstructing the digestive tract, the surgeon can eliminate the tumor while preserving the patient’s ability to digest food.
Although total gastrectomy can be life-saving, it significantly alters the digestive process and requires permanent lifestyle adjustments. The patient will need to adapt to new eating habits, manage potential side effects and work closely with their healthcare team to maintain optimal nutrition and overall health.
What is the recovery process like after total gastrectomy for stomach cancer?
Full healing from total gastrectomy is a gradual process that can take up to several months. With proper care and follow-up, the patient can successfully adapt to life without a stomach. Key aspects of the recovery process include:
- A hospital stay – Most patients remain in the hospital for one to two weeks for post-operative monitoring, pain management and nutritional support via IV or a feeding tube.
- Dietary changes – To accommodate the resulting digestive changes, the patient will start with liquids, progress to soft foods and then adopt a long-term diet of small, frequent meals.
- Activity restrictions – While the patient should avoid heavy lifting and other strenuous physical activities for six to eight weeks, they will be encouraged to engage in light activities, such as walking, soon after surgery to aid their recovery.
- Nutritional support – Lifelong vitamin B12 injections and iron and calcium supplements may be needed to prevent deficiencies.
- Gastrointestinal symptoms – Nausea and diarrhea are common and can usually be managed by avoiding sugary foods, eating small portions and resting after meals.
- Body weight and appetite changes – Because significant weight loss and reduced appetite are common after total gastrectomy, the patient will need to work with a dietitian to maintain balanced nutrition.
- Follow-up care – The patient will have regular checkups, imaging tests and blood work to monitor their recovery and check for signs of cancer recurrence.
What are the risks and possible complications of total gastrectomy for stomach cancer?
Total gastrectomy is a complex surgical procedure that carries several risks and potential complications due to its significant impact on the patient’s digestion and overall health. These include:
- Infection – There is a risk of infection at the incision site or inside the abdomen, which may require antibiotics or further treatment.
- Bleeding – Blood loss during or after surgery may necessitate a transfusion.
- Leakage – Seepage from the surgical connection between the esophagus and small intestine may require additional intervention.
- Nutritional deficiencies – Changes in digestion can lead to long-term deficiencies in vitamin B12, iron and calcium, which may require lifelong supplementation.
- Dumping syndrome – Rapid emptying of food into the intestines can cause gastrointestinal symptoms, such as nausea, cramping and diarrhea.
- Delayed gastric emptying – Slow movement of food through the digestive tract can cause nausea and bloating.
- Weight loss – Due to the resulting changes in digestion, significant weight loss and reduced appetite are common after total gastrectomy.
- Strictures – Scar tissue formation at the surgical site can cause narrowing in the digestive tract and may require dilation or additional surgery.
- Blood clots – Surgery increases the risk of deep vein thrombosis (DVT) and pulmonary embolism, especially during the recovery period.
- Tiredness – Fatigue and weakness are common during recovery from total gastrectomy due to the resulting dietary changes and reduced calorie intake.

Hyperthermic (heated) intraperitoneal chemotherapy (HIPEC) for stomach cancer
HIPEC is a specialized treatment that combines surgery with a concentrated dose of heated chemotherapy to directly target cancer cells in the abdominal cavity. This combined approach can improve the outcome and quality of life for a patient with advanced stomach cancer, particularly when the tumor has spread to the lining of the abdomen (peritoneal cavity) and treatment options are limited.
The procedure begins with cytoreductive surgery to remove all visible cancer cells from the peritoneal cavity. Immediately after the tumor is removed, a heated chemo solution will be circulated within the abdominal cavity for 60 to 90 minutes. Heat can enhance the effectiveness of chemotherapy by increasing its penetration into the abdominal tissues, improving its ability to destroy cancer cells. After the solution is drained, the surgical site will be closed.
HIPEC can be effective in controlling peritoneal cancer spread, particularly in cases where systemic chemotherapy alone has shown limited success. It may also reduce the risk of cancer recurrence by targeting microscopic tumor cells left behind after surgery. Currently available only at specialized cancer centers, this complex treatment may be considered for a patient who is healthy enough to tolerate the procedure and likely to benefit from it.
What is the recovery process like after hyperthermic intraperitoneal chemotherapy for stomach cancer?
Recovery after HIPEC is a gradual process that involves healing from both the surgery and the chemotherapy. While the procedure can be highly effective in managing advanced stomach cancer, it requires a comprehensive follow-up plan to address the patient’s physical and nutritional needs. Full recovery can take up to several months. During that time, the patient can expect:
- A hospital stay – Most patients remain in the hospital for one to two weeks for monitoring and pain management.
- Pain and fatigue – Due to the intensity of the treatment, discomfort is common and can usually be managed with medication, although the patient may feel tired for several weeks.
- Wound care – To help prevent infection, the patient should closely follow their physician’s instructions on how to properly care for the surgical incisions.
- Digestive recovery – Because temporary changes in appetite, bowel function and digestion are common after HIPEC, the patient may need to follow a special diet during recovery.
- Nutritional support – A dietitian can help the patient manage their nutritional needs, particularly if weight loss or vitamin deficiencies occur post-treatment.
- Activity restrictions – Early on, the patient will be encouraged to engage in light activities, such as walking, but they will need to avoid strenuous activities for six to eight weeks.
- Immune system monitoring – Because chemotherapy can temporarily weaken the immune system, the patient may be more susceptible to infection during recovery.
- Counseling and support – The physical toll of HIPEC can impact the patient’s emotional well-being; therefore, counseling and support groups may be beneficial.
- Follow-up care – The patient will have regular appointments with their oncology team to monitor healing, manage side effects and check for signs of cancer recurrence.
What are the risks and possible complications of hyperthermic intraperitoneal chemotherapy for stomach cancer?
HIPEC is a complex and intensive treatment that carries certain risks and potential complications. A thorough understanding of these risks is essential for a patient who is considering the procedure.
The primary risks and complications associated with hyperthermic intraperitoneal chemotherapy include:
- Infection – With HIPEC, surgical site infections and abdominal infections are possible and may require antibiotics or additional treatment.
- Bleeding – Significant blood loss during the surgical component may necessitate a blood transfusion.
- Organ damage – Nearby organs, such as the intestines, liver and kidneys, may be inadvertently injured during the surgery.
- Chemotherapy toxicity – The high dose of chemotherapy may cause side effects, such as nausea and vomiting.
- Fluid imbalances – The heated chemotherapy solution and surgical stress may lead to fluid shifts, potentially causing dehydration or swelling.
- Blood clots – As with any surgery, there is an increased risk of deep vein thrombosis (DVT) and pulmonary embolism during recovery.
- Bowel obstruction – Scar tissue or inflammation from the procedure may lead to an intestinal blockage.
- Nutritional challenges – Temporary or long-term difficulties with digestion and nutrient absorption may arise, requiring dietary adjustments.
- Fatigue and weakness – The prolonged recovery and combined effects of surgery and chemotherapy often result in extreme tiredness.
- Cancer recurrence – While HIPEC can be highly effective, cancer may recur in the peritoneal cavity or develop elsewhere in the body.
ACS Surgical Quality Partner for 30+ Years
Continuously Accredited by the American College of Surgeons Commission on Cancer since 1989 for our commitment to providing comprehensive, high-quality and multispecialty patient-centered care.
Surgical options for unresectable stomach cancer
If a stomach tumor is inoperable due to its size, location or advanced stage, surgical treatment may be considered to improve the patient’s comfort and quality of life. The goal is to address any tumor-related complications, such as digestive obstruction, bleeding and difficulty swallowing. Two common treatment approaches include:
Stent placement for unresectable stomach cancer
Stent placement is a minimally invasive technique that can be used to alleviate symptoms caused by a stomach tumor, such as a blockage that prevents food or liquids from passing through the digestive tract. During the procedure, a surgeon will endoscopically insert a self-expanding metal stent into the obstructed area to reopen the passage. This can help restore normal digestion and provide immediate relief from nausea, vomiting and difficulty eating.
This surgery can be particularly beneficial for a patient with advanced-stage stomach cancer or a nonresectable tumor. However, it is important to note that stent placement will not treat the cancer itself. Instead, the procedure is usually performed as part of a broader supportive care plan.
What is the recovery process like after stent placement for unresectable stomach cancer?
Recovery after stent placement for stomach cancer is typically quicker and less intensive compared to other surgical treatments. The minimally invasive nature of the procedure allows most patients to resume their normal activities within a few days. Key aspects of the recovery process include:
- No overnight hospitalization – After a short period of observation in the hospital, most patients can return home the same day.
- Dietary adjustments – After starting with liquids and soft foods, the patient may gradually transition to solid foods as tolerated.
- Symptom relief – Many patients experience significant symptom improvement immediately after the procedure.
- Pain management – Mild discomfort or soreness at the stent insertion site is common and can usually be managed with over-the-counter pain relievers.
- Quick activity resumption – Normal activities can usually be resumed within a few days, but the patient should avoid strenuous activities for a few weeks.
- Monitoring – Regular follow-up appointments will be scheduled to ensure the stent remains functional and address any surgical complications.
What are the risks and possible complications of stent placement for unresectable stomach cancer?
While stent placement for unresectable stomach cancer is a minimally invasive procedure with significant benefits, it does carry some risks and potential complications. These include:
- Stent migration – The stent may drift from its original position, leading to the recurrence of symptoms or the need for stent repositioning.
- Stent blockage – Tumor growth or food debris may obstruct the stent, requiring further intervention to restore its functionality.
- Tissue perforation – Rarely, the stent may cause a tear in the stomach or intestinal wall, which can lead to infection and may require emergency treatment.
- Bleeding – Minor bleeding may occur at the stent site, though significant bleeding is uncommon.
- Pain – Some patients experience discomfort at the stent site, which may persist and require pain management.
- Indigestion – Stent placement can lead to acid reflux or heartburn, particularly if the stent is near the stomach entrance.
- Infection – There is a small risk of infection following the procedure, which may require antibiotics.
Endoscopic tumor ablation for unresectable stomach cancer
Endoscopic tumor ablation is a minimally invasive technique that can be used to reduce the size of an unresectable stomach tumor that is interfering with digestion. Using an endoscope, the surgeon will deliver heat, cold or laser energy directly to the tumor, destroying the cancerous cells. This treatment can be particularly beneficial for alleviating tumor-related symptoms, such as obstruction, bleeding or difficulty swallowing, potentially offering relief to a patient who may not be a candidate for traditional stomach cancer surgery.
While endoscopic tumor ablation does not cure stomach cancer, it can greatly improve the patient’s quality of life. The procedure can be repeated if necessary and is typically part of a broader treatment plan that may include other therapies, such as chemotherapy.
What is the recovery process like after endoscopic tumor ablation for unresectable stomach cancer?
The recovery process after endoscopic tumor ablation is generally quick, with many patients able to return to their normal activities within a few days. Because the surgery is minimally invasive, it typically involves less pain and a faster recovery compared to more invasive stomach cancer procedures. As the patient’s body heals and adjusts, they can generally expect:
- A short hospital stay – Most patients can go home the same day or after a brief observation period in the hospital.
- Dietary modifications – Soft foods and liquids are typically recommended initially, followed by a gradual return to a normal diet as tolerated.
- Pain management – Mild discomfort or abdominal bloating may occur, but these symptoms can usually be managed with over-the-counter pain relievers.
- Quick resumption of daily activities – Light activities, such as walking, can be resumed soon after the procedure, but strenuous activities should be avoided for a few days.
- Symptom relief – Many patients experience immediate improvement in their tumor-related symptoms, such as nausea and difficulty swallowing.
- Follow-up care – Regular follow-up appointments will be important to monitor the effectiveness of the treatment and check for complications, such as bleeding and symptom recurrence.
What are the risks and possible complications of endoscopic tumor ablation for unresectable stomach cancer?
Endoscopic tumor ablation is generally considered safe, but like any medical treatment, it carries some risks and potential complications. These include:
- Bleeding – Though rare, excessive bleeding can occur at the treatment site, which may require intervention.
- Discomfort – Some patients experience abdominal pain and bloating after the procedure.
- Infection – There is a slight risk of infection at the endoscope insertion site or internally, which may require antibiotic therapy.
- Tissue perforation – The procedure may cause a tear in the stomach or nearby tissue, which may lead to serious complications that necessitate surgical intervention.
- Stricture formation – Scar tissue can develop at the treatment site, which may narrow the stomach or digestive tract and require further treatment.
- Symptom recurrence – In some cases, the tumor may not be completely ablated or the symptoms may return, requiring additional treatment.
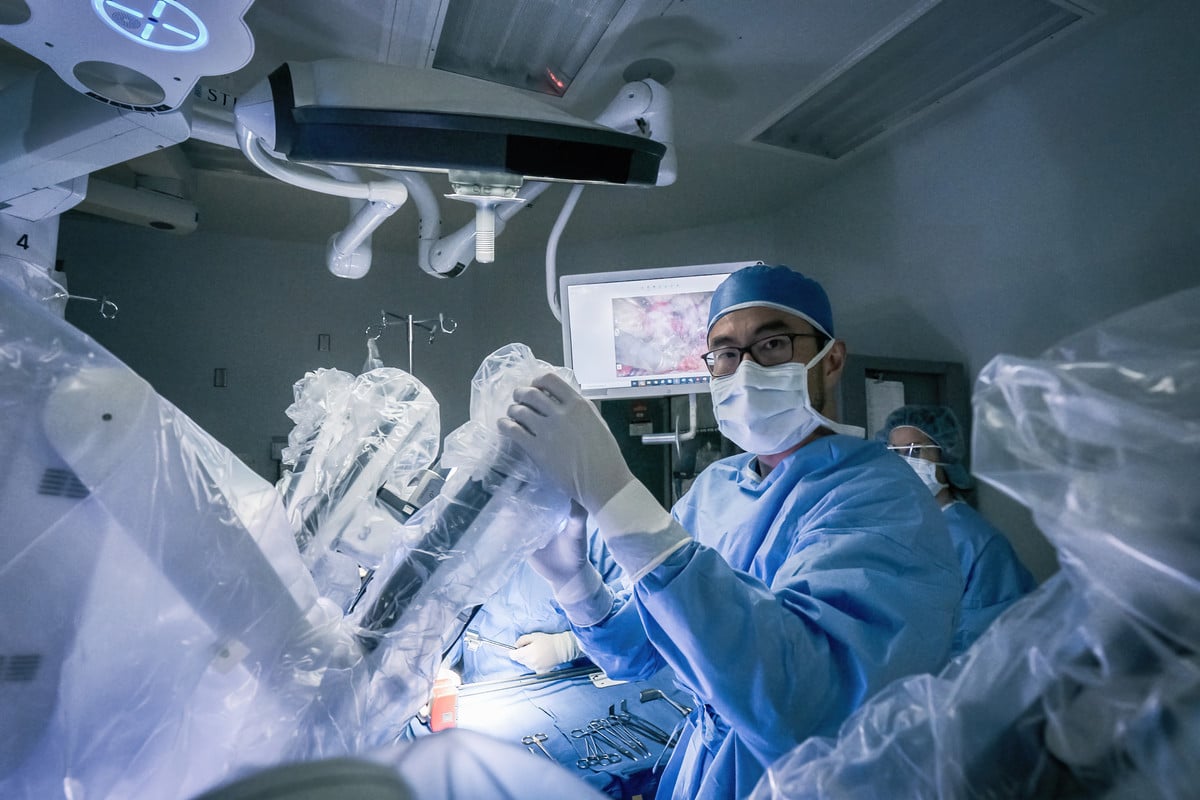
Robotic surgery for stomach cancer
Robotic surgery is a minimally invasive technique that involves the use of an advanced robotic system, such as the da Vinci® Surgical System, to achieve heightened surgical precision. While operating, the surgeon can maneuver and control robotic instruments via a computer console, allowing for smaller incisions, greater dexterity and a more detailed view of the surgical area. This technology may be used for both curative and supportive stomach cancer treatments, including tumor removal, partial gastrectomy and lymph node dissection.
Compared to traditional open surgery, robotic surgery offers several key advantages, including less blood loss, smaller scars and a faster recovery. It also allows for more precise dissections, particularly in difficult-to-reach areas of the abdominal cavity. However, as with any surgery, robotic surgery carries certain risks, such as infection, excessive bleeding and anesthesia complications.
A surgeon may use robotic assistance when performing several types of stomach cancer surgery, including:
- Partial gastrectomy – Removes the portion of the stomach affected by cancer while preserving the remaining healthy tissue to maintain digestive function
- Total gastrectomy – Removes the stomach in its entirety (often necessary for advanced or widespread stomach cancer) with reconstruction of the digestive tract
- Wedge gastric resection – Excises a small, localized section of the stomach, typically for treating an early-stage or noninvasive tumor
- Transgastric tumor resection – Removes a tumor located inside the stomach by accessing it directly through the stomach wall
Radiation therapy for stomach cancer
A common treatment for stomach cancer, radiation therapy uses high-energy beams to destroy cancerous cells and shrink tumors. It can be used as a standalone treatment, in combination with surgery or chemotherapy or as a supportive care option to relieve tumor-related symptoms, such as pain, bleeding and difficulty eating. The goal is to target the cancerous tissues without affecting the surrounding healthy structures.
External beam radiation therapy (EBRT) for stomach cancer
EBRT uses high-energy beams directed at a tumor from outside the body. Advanced imaging techniques may be used to map the tumor and surrounding tissues, ensuring the beams will be precisely focused on the cancerous cells while sparing nearby structures, such as the liver and intestines.
The types of external beam radiation therapy for stomach cancer include:
- Three-dimensional conformal radiation therapy (3D-CRT) – 3D-CRT uses advanced imaging technology to shape the radiation beams to the exact dimensions of the tumor, minimizing exposure to surrounding healthy tissues.
- Intensity-modulated radiation therapy (IMRT) – IMRT delivers radiation therapy in precise doses by modulating the intensity of the beam, allowing for greater control over the amount delivered to the tumor.
- Image-guided radiation therapy (IGRT) – IGRT combines imaging technologies with radiation therapy, ensuring highly accurate targeting of the tumor by continually adjusting for any movement or changes in the position of the tumor.
- Stereotactic radiosurgery (SRS) – Despite its name, SRS is a nonsurgical procedure that delivers high-dose, precisely targeted radiation therapy to a small, well-defined tumor in a few sessions.
EBRT is often used in combination with chemotherapy for enhanced treatment effectiveness or after surgery to eliminate any remaining microscopic cancer cells. Typically, it is delivered over several sessions spread across a few weeks to allow healthy tissues time to recover.
What are the potential benefits of external beam radiation therapy for stomach cancer?
EBRT offers several potential benefits for patients with stomach cancer, particularly when used as part of a comprehensive treatment plan. By delivering high-energy beams directly to a tumor, external beam radiation therapy can help control cancer progression, alleviate tumor-related symptoms and improve the patient’s quality of life. Key advantages of EBRT include:
- Tumor reduction – EBRT can shrink a tumor, making it more manageable or easier to remove during surgery.
- Symptom relief – Radiation therapy can alleviate symptoms caused by the tumor, such as pain, bleeding and obstruction.
- High level of precision – By focusing the high-energy beams precisely on the tumor, EBRT minimizes exposure to surrounding healthy tissues.
- Post-surgical support – After surgery, radiation therapy can eliminate residual cancer cells, reducing the risk of recurrence.
- Combined effectiveness – EBRT can enhance the impact of chemotherapy when used concurrently as part of a comprehensive treatment approach.
- Noninvasive technique – Radiation therapy may be a good treatment option for a patient who is not a candidate for surgery due to health concerns or advanced cancer.
What are the possible side effects and complications of external beam radiation therapy for stomach cancer?
EBRT is a powerful treatment that can cause temporary side effects and complications due to its impact on healthy tissues. These include:
- Fatigue – Many patients feel increasingly tired as the treatment progresses.
- Nausea and vomiting – Radiation treatment to the stomach area can irritate the gastrointestinal system, leading to temporary digestive issues.
- Diarrhea – Increased bowel sensitivity may result in loose stools or more frequent bowel movements.
- Skin changes – Redness, irritation or sensitivity can occur at the treatment site, similar to a mild sunburn.
- Loss of appetite – Treatment-related nausea or changes in digestion can affect appetite and food intake.
- Organ damage – Rarely, radiation therapy may impact nearby organs, such as the liver, kidneys or intestines, causing additional complications.
- Scarring or stiffness – Long-term effects of radiation therapy can include scarring or stiffness in the treated area, potentially affecting digestive function.
- Emotional impact – Stress or anxiety related to cancer treatment can affect mental well-being and may require emotional support.
Internal radiation therapy for stomach cancer
Internal radiation therapy, also known as brachytherapy, is a localized treatment for stomach cancer that involves the temporary or permanent placement of a small radioactive device inside or near a tumor. This concentrated approach delivers a high dose of radiation therapy directly to the cancerous cells.
High-dose-rate brachytherapy is typically used for advanced or inoperable stomach cancer to shrink the tumor and reduce the related symptoms, often as part of a comprehensive supportive care plan. While this radiation delivery technique potentially offers higher precision and fewer side effects than external beam radiation therapy, it requires specialized expertise and is not suitable for all patients.
What are the potential benefits of internal radiation therapy for stomach cancer?
Compared to EBRT, brachytherapy offers several potential advantages for treating stomach cancer. These include:
- Enhanced precision – Brachytherapy delivers radiation treatment directly to a tumor, minimizing exposure to surrounding organs and tissues.
- Improved comfort – Internal radiation therapy can relieve symptoms, such as pain and bleeding, caused by advanced stomach cancer.
- Higher dose – Brachytherapy allows for the delivery of a higher dose of radiation therapy, potentially improving treatment effectiveness.
- Shorter treatment duration – Typically, brachytherapy requires fewer treatment sessions, saving time and reducing patient fatigue.
What are the possible side effects and complications of internal radiation therapy for stomach cancer?
Brachytherapy is a highly targeted treatment for stomach cancer, but it can still cause side effects and complications due to its effect on nearby tissues. These include:
- Localized discomfort – Temporary irritation or soreness may develop at the device insertion site.
- Nausea and vomiting – Radiation therapy delivered to the stomach may cause digestive upset, particularly during or immediately after treatment.
- Bowel changes – Diarrhea or constipation can occur due to irritation of the gastrointestinal tract.
- Fatigue – Many patients feel tired both during and immediately after treatment.
- Infection – Rarely, the device insertion site can become infected and require medical attention.
- Scarring – Long-term exposure to radiation therapy may lead to minor scarring or stiffness in the treated area.
- Blood loss – Minor bleeding at the treatment site is possible, especially in cases where the tumor is located near blood vessels.
Systemic radiation therapy for stomach cancer
Systemic radiation therapy involves the use of liquid radioactive drugs (radiopharmaceuticals) that enter the bloodstream and circulate throughout the body. Radiopharmaceuticals such as radioactive isotopes are designed to selectively bind to tumor cells, delivering radiation therapy directly to the cancerous tissues.
An example of systemic radiation therapy is radioactive iodine therapy, which involves the oral or intravenous administration of radioactive iodine. Although this treatment is primarily used for hypothyroidism and thyroid cancer, other types of systemic radiation therapy may be considered in select cases of advanced or metastatic stomach cancer.
What are the potential benefits of systemic radiation therapy for stomach cancer?
Systemic radiation therapy is a unique treatment approach for advanced or metastatic stomach cancer. Potential benefits include:
- Whole-body targeting – Because systemic radiation therapy can target cancerous cells throughout the body, it can be particularly effective for metastatic stomach cancer.
- Precision binding – Radiopharmaceuticals selectively bind to cancer cells, minimizing damage to healthy tissues.
- Symptom relief – Systemic radiation therapy can alleviate symptoms caused by widespread cancer, such as bone pain and organ dysfunction.
- Combination therapy – When used in conjunction with other treatments, such as chemotherapy, systemic radiation therapy can better control the progression of stomach cancer.
- Minimally invasive treatment – Administered orally, intravenously or via injection, systemic radiation therapy may help a patient avoid invasive surgery.
What are the possible side effects and complications of systemic radiation therapy for stomach cancer?
Systemic radiation therapy can cause side effects due to its impact on both cancerous and healthy tissues. These include:
- Fatigue – Due to the body’s response to the radiation therapy and its impact on healthy tissues, the patient may feel weak and tired.
- Digestive issues – Nausea, vomiting, diarrhea or constipation may occur, especially if the radiation treatment affects the gastrointestinal system.
- Low blood cell counts – Radiation therapy can interfere with the body’s production of healthy blood cells, leading to an increased risk of infection, anemia and bleeding.
- Skin irritation – If the treatment is administered via injection, mild redness or irritation may occur at the injection site.
- Pain at the tumor site – Occasionally, systemic radiation therapy may cause discomfort in areas of the body where cancer cells are being targeted.
- Long-term effects – Systemic radiation therapy can potentially cause organ damage, particularly if the treatment affects sensitive tissues over time.
Radiation therapy for advanced stomach cancer
For advanced or metastatic stomach cancer, radiation therapy may be used as a supportive treatment to manage the symptoms of an inoperable tumor and improve the patient’s quality of life. Depending on the location and extent of the tumor, external beam radiation therapy, brachytherapy or systemic radiation therapy may be considered.
While radiation therapy is typically not curative for advanced-stage stomach cancer, it can shrink a tumor and alleviate the related pain, bleeding and blockages. Usually, it is used in combination with chemotherapy or other treatments to reduce the tumor burden and support the patient’s overall treatment plan.
Chemotherapy for stomach cancer
A widely used treatment for stomach cancer, chemotherapy involves the administration of a custom blend of powerful drugs to destroy cancer cells or inhibit their growth. Most chemo drugs are delivered via IV infusion, although some can be swallowed in pill or capsule form.
For early-stage stomach cancer, chemotherapy may be used in combination with other treatments, such as surgery or radiation therapy, for heightened effectiveness. For advanced or metastatic stomach cancer, chemo may be the primary treatment to control tumor growth, alleviate symptoms and improve the patient’s quality of life.
What are the potential benefits of chemotherapy for stomach cancer?
Chemotherapy is an important treatment option for stomach cancer, offering benefits that can vary depending on the stage and progression of the tumor. These include:
- Tumor shrinkage – Chemo can reduce the size of a tumor, making it easier to remove with surgery or manage with other therapies.
- Improved surgical outcome – When administered before surgery, chemotherapy can increase the likelihood of successful tumor removal.
- Elimination of microscopic cancer cells – When administered after surgery, chemotherapy can help destroy any remaining cancer cells to reduce the risk of recurrence.
- Symptom relief – By controlling stomach cancer growth, chemo can alleviate tumor-related symptoms, such as pain, bleeding and obstructions.
- Effective treatment of advanced cancer – Chemotherapy is a systemic treatment that targets rapidly dividing cells throughout the body, which can be particularly beneficial for treating metastatic and inoperable tumors.
- Combination therapy – When used as part of a multimodal treatment approach, chemotherapy can enhance the effectiveness of radiation therapy and other therapies.
What are the possible side effects and complications of chemotherapy for stomach cancer?
Chemotherapy can be an effective treatment for stomach cancer. However, due to the impact of the chemo drugs on both cancerous and healthy cells, it has several possible side effects and complications. These include:
- Digestive issues – Many chemo drugs can irritate the gastrointestinal system and disrupt normal digestion, causing symptoms such as nausea, vomiting, diarrhea or constipation.
- Fatigue – Chemotherapy is an intensive treatment that often leads to extreme tiredness.
- Hair loss – Certain chemo drugs can damage rapidly dividing hair follicle cells, resulting in temporary hair loss.
- Loss of appetite – Chemotherapy can alter taste and cause nausea, leading to reduced appetite.
- Mouth sores – Some chemo drugs can irritate the lining of the mouth, leading to painful sores and difficulty eating and drinking.
- Low blood cell counts – Chemo can suppress the production of healthy blood cells, increasing the risk of infection, anemia and excessive bleeding.
- Neuropathy – Some chemo agents can irritate nerves, leading to numbness, tingling sensations or pain, especially in the hands and feet.
- Emotional and mental strain – The physical toll of chemotherapy can contribute to anxiety, depression and other psychological challenges.
Molecular therapy for stomach cancer
Molecular therapy, also known as targeted therapy, is an innovative treatment approach for stomach cancer that focuses on specific genetic or molecular changes in tumor cells. Unlike traditional chemotherapy, which affects both cancerous and healthy cells, molecular therapy is tailored to the patient’s unique tumor profile, aiming to block the pathways that allow cancer cells to grow and spread.
Common types of molecular therapy for stomach cancer include drugs that target HER2, a protein involved in cell growth, or angiogenesis, the formation of new blood vessels that supply tumors. By disrupting these processes, molecular therapy can slow cancer progression, enhance the effectiveness of other treatments and, in some cases, lead to a better outcome and quality of life. As research advances, molecular therapy continues to offer promising, less invasive treatment options for stomach cancer.
What are the potential benefits of molecular therapy for stomach cancer?
A form of precision medicine, molecular therapy minimizes harm to healthy cells while effectively disrupting cancer growth and progression. Potential benefits include:
- Precise tumor targeting – Molecular therapy specifically attacks cancer cells with particular genetic mutations or proteins, reducing damage to healthy tissues.
- Improved treatment effectiveness – By focusing on a tumor’s unique characteristics, molecular therapy can lead to a better outcome and quality of life.
- Reduced side effects – Because molecular therapy avoids nonspecific damage to healthy cells, it generally has fewer side effects than chemotherapy.
- Combination therapy potential – Targeted therapies can be combined with chemotherapy or other treatments to improve overall efficacy.
- Cancer progression control – Molecular therapy can slow tumor growth and reduce the likelihood of metastasis in cases of advanced stomach cancer.
- Personalized treatment – Molecular therapy allows for a customized treatment plan based on the patient’s specific tumor profile.
What are the possible side effects and complications of molecular therapy for stomach cancer?
While effective in targeting cancer cells, molecular therapy can cause side effects and complications due to its impact on specific pathways in the body. These include:
- Fatigue – Many patients feel tired as their body responds to the therapy.
- Nausea and vomiting – Some targeted therapies may irritate the gastrointestinal system or disrupt normal digestive processes, causing nausea, vomiting or diarrhea.
- Skin reactions – Some patients develop rashes, dry skin or other dermatological issues, particularly with therapies that target specific growth factors.
- Hypertension – Some molecular therapies that target blood vessel growth may cause elevated blood pressure.
- Liver toxicity – Molecular therapy can sometimes lead to liver inflammation and impaired liver function, which require close monitoring.
- Bleeding or blood clots – Certain targeted treatments may increase the risk of abnormal bleeding or clot formation.
- Allergic reactions – Some patients may experience allergic or infusion-related reactions during treatment.
- Infection risk – Although less common than with chemotherapy, targeted therapy may suppress the immune system, increasing vulnerability to infections.
Stomach cancer clinical trials
Clinical trials are important research studies designed to help scientists and clinicians evaluate the effectiveness of newly developed treatments, such as novel chemotherapy regimens, radiation delivery techniques, surgical procedures and targeted therapies. These studies play an invaluable role in advancing stomach cancer care, providing patients with unique opportunities to benefit from groundbreaking therapies that are not yet available in other settings. They also serve as a source of hope to certain patients with stomach cancer, particularly those with advanced, metastatic or treatment-resistant tumors.
The Moffitt Cancer Center difference in stomach cancer treatment
Moffitt is one of the largest and highest-volume cancer centers in the nation, and the surgeons in our Gastrointestinal Oncology Program have extensive experience in performing complex procedures, including partial and total gastrectomies. We also offer the latest chemotherapy drugs and radiation delivery techniques for stomach cancer. Additionally, through our robust portfolio of clinical trials, our patients can benefit from emerging treatment options that are not yet widely available.
Moffitt stands apart from other cancer centers due to the world-class expertise of our multidisciplinary team and our commitment to delivering evidence-based, highly individualized cancer care. If you would like to explore your treatment options for stomach cancer with a specialist at Moffitt, you can request an appointment by calling 1-888-663-3488 or using our online form. We do not require referrals.
Helpful links:

