Moffitt Patient Is First To Receive Second CAR T Therapy for Ovarian Cancer
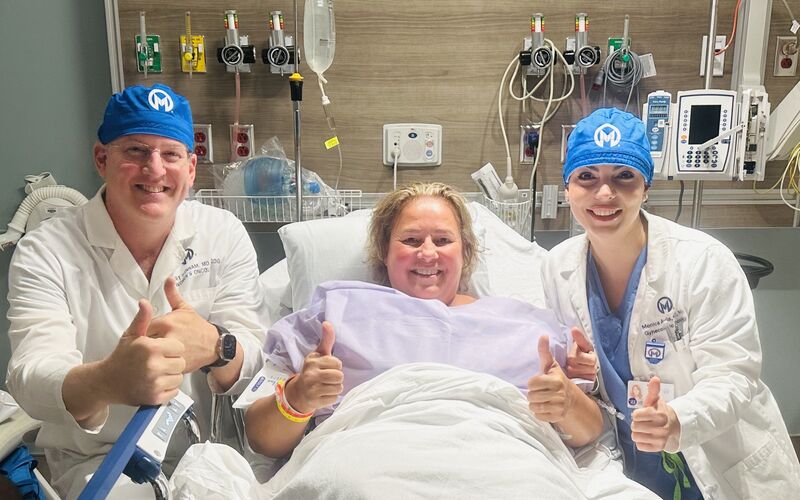
With her husband by her side, Marisol Gallagher patiently waits inside her hospital room at Moffitt Cancer Center. Her cells are due to arrive in a few hours — T cells that had been removed from her body and mixed with a specific protein in the lab.
This wasn’t the first time Gallagher sat waiting for a treatment called chimeric antigen receptor therapy, or CAR T-cell therapy. She received the therapy in June 2023 as part of a trial testing CAR T in ovarian cancer. But this was the first time in the world an ovarian cancer patient was undergoing CAR T a second time after Moffitt secured Food and Drug Administration approval to open a trial just for Gallagher.
“This is uncharted territory,” said Monica Avila, MD, Gallagher’s gynecologic oncologist. “This is still so new, and we are figuring it out.”

CAR T is a cellular immunotherapy that uses the body’s own immune system to attack cancer. A patient’s T cells are harvested from their body, genetically engineered in a lab and infused back into the patient. It is currently only approved for patients with certain blood cancers. While it’s being studied in solid tumors, it hasn’t been as successful.
“One of the criticisms of solid tumor CAR T is that it’s difficult because there are a lot of different types of cells we are trying to tackle in one big ball,” Avila said. “We still don’t know if the treatment is getting into all of those sites.”
For ovarian cancer patients, their T cells are engineered to target the follicle stimulating hormone receptor that is exclusively present on ovarian cells. The genetically engineered cells are then delivered directly into a patient’s abdomen via a surgically placed catheter.
The experimental treatment gave new hope to Gallagher, who had relapsed four times since her diagnosis in 2014. She was working as a nurse and had just celebrated her 50th birthday when she learned her gastrointestinal issues were actually being caused by ovarian cancer. She immediately began a revolving door of chemotherapies and surgeries.
“It just kept relapsing and each time the remission time was shorter and shorter,” Gallagher said. “I didn’t qualify for any trials at the time because I had received so much chemotherapy.”
Gallagher’s care team where she lived in New Jersey told her she was out of options and to live out the time she had left the best she could. However, when she moved to Florida, she sought a second opinion at Moffitt. She found out she was a candidate for CAR T and was one of the first patients to enroll on the groundbreaking trial led by Robert Wenham, MD, chair of Moffitt’s Gynecologic Oncology Department.

Robert Wenham, MD
“Being able to write and lead a study where we are directing a patient’s immune cells to target their cancer is as exciting as it gets,” Wenham said. “While we have made some gains for patients with more chemotherapy and drugs, it has been incremental. The big breakthroughs in survival, and I dare say cure, will come from harnessing the patient’s immune system; something I and our entire team at Moffitt are fully committed to solving.”
Gallagher did well throughout the process. After receiving the treatment, however, her tumor appeared to be growing slightly on scans. Despite this, she was feeling great and she and her doctors decided to hold off on starting any treatment. Over time, her tumor began to shrink.
“The treatment also prevented any new cancer growth. I should have had more tumors pop up. Every time it came back, it came back stronger. This stopped that,” Gallagher said.
Gallagher could now go out with friends, enjoy time on her boat fishing and visit her daughter who works as a nurse in Philadelphia.
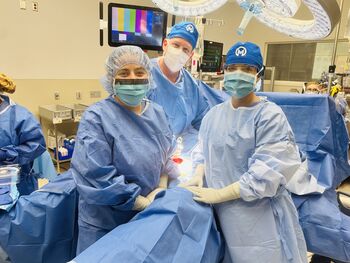
Drs. Robert Wehnam and Monica Avila surgically placed a peritoneal catheter into Marisol Gallagher's abdomen. The catheter is used to infuse Gallagher's second round of cells into her body to attack her cancer.
But with one tumor left and the presumption of more microscopic disease, Gallagher’s care team wanted to see if they could do more. Because the tumor was covered by Gallagher’s intestine, it would be very difficult to surgically remove. Since she responded so well to CAR T the first time, what if they tried again?
After securing FDA approval for the first time to give an ovarian cancer patient a second round of CAR T, Gallagher started the process again with the harvesting of new cells.
“It will be interesting to see if these cells have adapted over time and are better equipped,” Avila said.
The cells were infused into Gallagher in October, 18 months after her first CAR T infusion.
“It was easier the second time,” Gallagher said. “I didn’t have any bad reactions that I did the first time.”
Almost two months later, she is still doing well. She is looking forward to enjoying the holidays with her family before her next follow-up visit in February.