New Drug Duo Significantly Slows Progression of Aggressive Breast Cancer
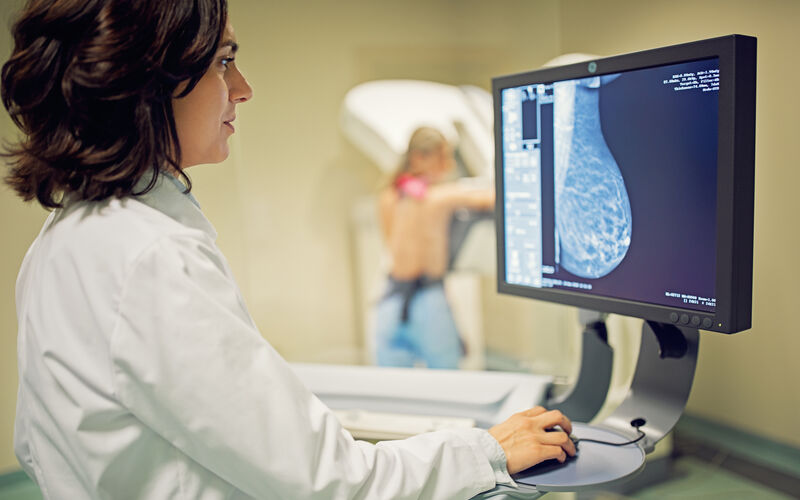
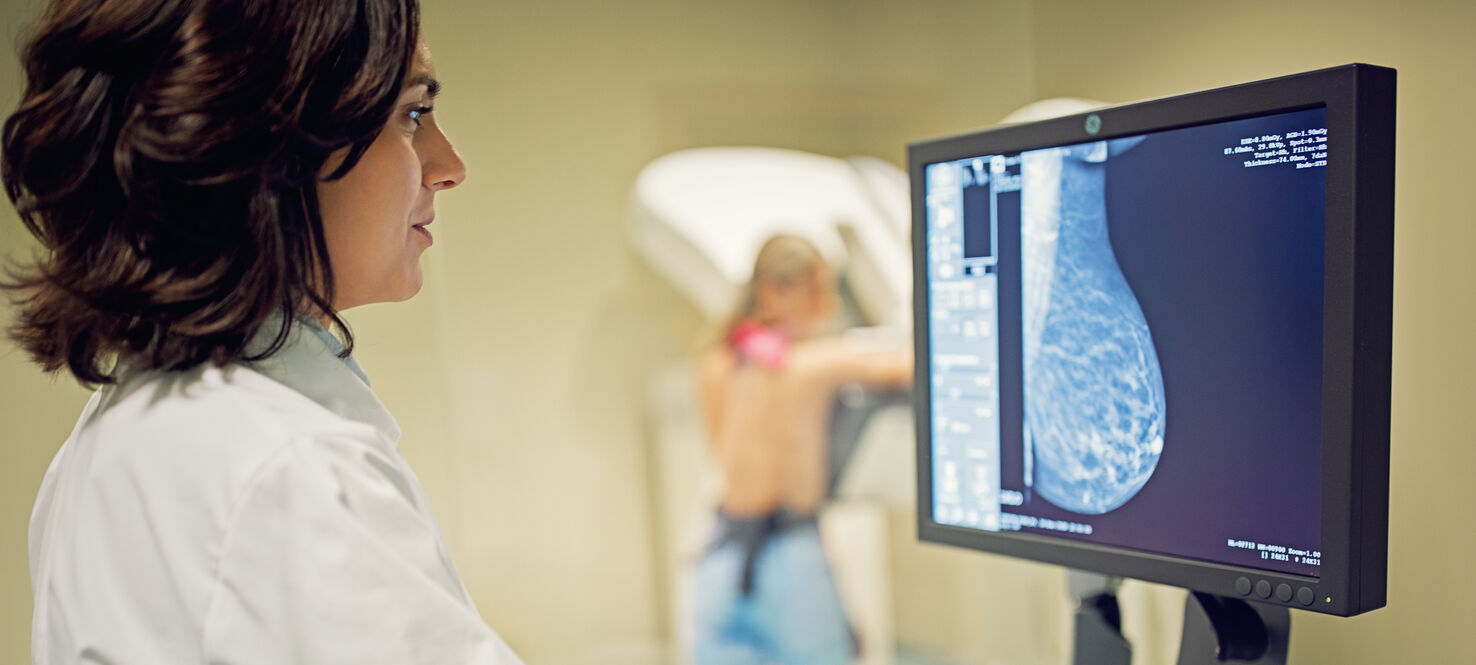
A promising new drug combination could offer a much-needed option for women battling one of the most aggressive forms of breast cancer. For the first time, a targeted therapy paired with immunotherapy has been shown to significantly delay the progression of advanced triple-negative breast cancer, outperforming the current standard treatment.
Triple-negative breast cancer accounts for roughly 10% of all breast cancer cases in the U.S. and is notoriously difficult to treat. Unlike other breast cancers, triple-negative breast cancer lacks three common receptors –– estrogen, progesterone and HER2 –– that many therapies target. This limits treatment options and increases the risk of recurrence. Even with recent advances, survival rates for patients with advanced, PD-L1-positive triple-negative breast cancer remain low, with only about one-third living beyond three years.
However, new findings from the phase 3 ASCENT-04/KEYNOTE-D19 clinical trial, presented at the American Society of Clinical Oncology annual meeting, offer new hope. The international study enrolled 443 patients across 26 countries and compared two treatment regimens: the experimental combination of sacituzumab govitecan, a targeted antibody-drug conjugate, and the immunotherapy pembrolizumab, versus the current standard of chemotherapy plus pembrolizumab.
After a median follow-up of 14 months, patients receiving the new drug pairing experienced a median progression-free survival of 11.2 months, compared to 7.8 months for those on standard therapy. The new combination reduced the risk of disease progression by 35% and nearly doubled the duration of response.

Hatem Soliman, MD
“This is a very positive development,” said Hatem Soliman, MD, a medical oncologist in the Breast Oncology Department at Moffitt Cancer Center. “We are always looking for ways to improve outcomes for women with metastatic triple-negative breast cancer.” He noted that while some clinicians have already used this combination off-label with encouraging results, the new trial provides the evidence needed to support broader adoption.
“A 35% reduction in the hazard ratio for progression is a substantial difference. This combination could very well replace the current standard of care,” Soliman added.
Sacituzumab govitecan is already approved as a later-line treatment for triple-negative breast cancer, but this is the first phase 3 trial to demonstrate its safety and efficacy as a first-line therapy when combined with pembrolizumab. Soliman emphasized the importance of using the most effective treatments early, while patients are still strong enough to tolerate them. “There’s a real push to put our best foot forward from the beginning,” he said.