Overcoming a Rare Cancer with Determination and a Revolutionary New Therapy
Thanksgiving is a time to gather with family and reflect on what you are thankful for. However, for JP Malherbe, the 2017 holiday changed his life forever.
Training for a half marathon, Malherbe got up on Thanksgiving and went for a run. He discovered a small lump on his right thigh during his post-run shower. He felt fine but still decided to get it looked at. After trips to an oncologist in his hometown in Kentucky and in Jacksonville, Fla., he was diagnosed with a rare type of cancer called synovial sarcoma. Little did he know that seven years later, Thanksgiving would again play a pivotal role in his life, marking the day he received a groundbreaking cell infusion that would change his cancer journey.
Synovial Sarcoma Fast Facts
- Synovial sarcoma is a rare and aggressive cancer that affects soft tissues.
- Approximately 1,000 new cases are diagnosed annually.
- It's most common in young adults and teenagers but can occur at any age.
- It's more common in males than females.
- Before Tecelra, the last FDA-approved therapy was pazopanib, an oral tyrosine kinase inhibitor approved in 2012. It has response rates of 5% to 10% and a response duration of about 5 months.
First-of-its-kind Opportunity
Synovial sarcoma is a soft tissue cancer that typically develops near large joints, most commonly around the knees. It is characterized by a slow-growing tumor that can originate in soft tissues such as muscles, ligaments or tendons. It is caused by an abnormal change in the chromosomes that can cause cells to grow uncontrollably, leading to tumor formation.
"We primarily see this type of sarcoma in adolescents and young adults," said Mihaela Druta, MD, a medical oncologist in Moffitt Cancer Center's Sarcoma Department. "It often presents as a painless lump or swelling, and if left untreated, can spread to other parts of the body, particularly the lungs."
Scans revealed Malherbe, who was 34 at the time, also had nodules on his lungs. In January 2018, he began a chemotherapy regimen known as the "red devil" because of the drug's color and harsh side effects. Then came radiation. By April, the tumor in his leg had shrunk enough for surgery. However, after removing the tumor — which left him with little function in his thigh — he developed an infection that resulted in an emergency hip replacement.
"I remember after surgery thinking I can't do anything fun. My life had changed. I went from a full, fast-paced life to completely slowed down because my body did not function as it used to," Malherbe said. "I could no longer run or ride horses. It hasn't been easy, but I always remember that there are others who are worse off than me. When I wake up each morning, I look in the mirror and tell myself, ‘You got this!’"
That positive attitude helped Malherbe as he continued his cancer journey. He went through physical therapy to improve his balance and ability to walk. And for the next several years, he would endure scans and treatment to monitor the nodules in his lungs. He has had three surgeries to remove cancerous tissue in his lungs.
"This type of cancer will always be with me. My oncologist has monitored my nodules, and I get treatment when they grow," Malherbe said.
However, in September 2024, he had reached a point where his lung nodules were growing, and he had run out of treatment options. He was facing palliative radiation therapy when fate stepped in. A family friend remembered a Moffitt oncologist talking about a new first-of-its-kind treatment for synovial sarcoma and recommended scheduling an appointment.
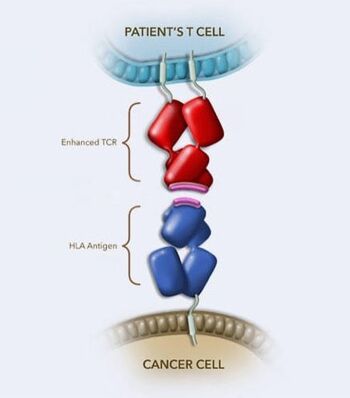
T-cell receptor (TCR) therapy uses enhanced TCRs to bind to HLA-presenting antigens on cancer cells, enabling T cells to kill them. Courtesy: AdaptImmune
The therapy the family friend was referring to is afamitresegene autoleucel, commercially known as Tecelra. It is a T-cell immunotherapy that uses a patient's own cells, which are genetically modified to target the MAGE-A4 antigen in synovial sarcoma cells. Moffitt was part of the clinical trials that led to the August 2024 approval by the U.S. Food and Drug Administration.
"Tecelra is the first engineered cell therapy for a solid tumor. It is a single infusion of cells that remain active in the bloodstream for long after the initial infusion, remaining functional for more than three years in some patients," Druta said.
Coincidentally, Malherbe had been screened for this therapy three years prior to meeting with Druta, when the cell therapy was still being evaluated in clinical trials but decided against the treatment at that time. "I didn't want to use the treatment unless I absolutely had to. It was always there as a last resort," he said.
Now, Malherbe was going to be the first person in the world to receive Tecelra post-FDA approval. Although treatment was slightly delayed by Hurricane Milton, Malherbe’s cells were collected in October and sent to a lab. His newly modified cells were returned to Moffitt in November.
"Before infusion, patients are given several days of chemotherapy to reduce the number of immune cells in the body. This makes room for the new modified cells to grow and expand within the body," Druta said.
A New Thanksgiving
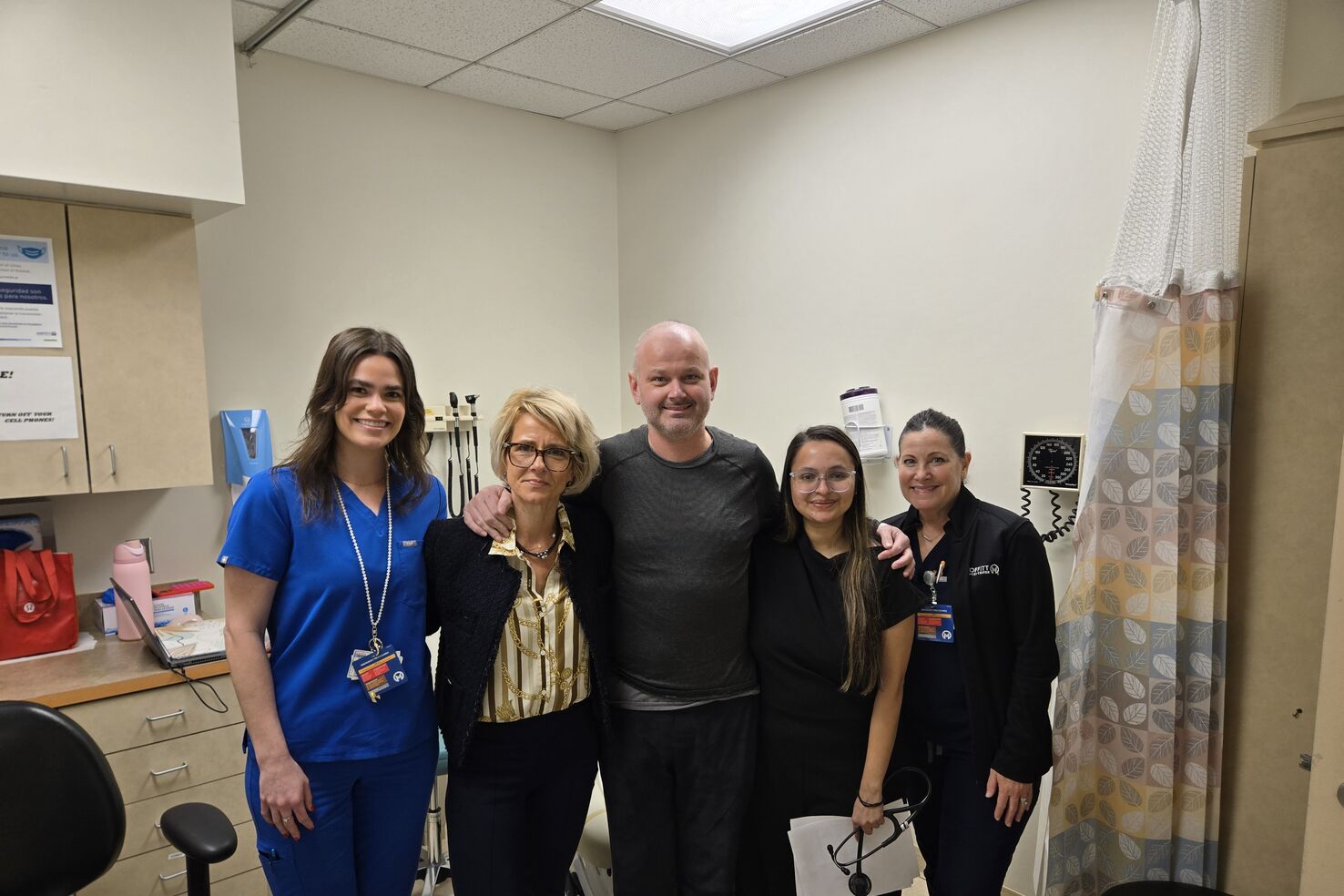
JP Malherbe, pictured with Dr. Mihaela Druta, received his infusion on Thanksgiving Day 2024.
Malherbe’s life changed again on Thanksgiving Day 2024, this time for the better. He finally received his modified cells to give him a better chance at a remission.
"The care team at Moffitt was wonderful and with me every step of the way. Dr. Druta was there for my infusion and spent several hours with me after to make sure I was OK," he said.
Malherbe recalls feeling fine the day of the infusion but later experienced side effects from the therapy. These side effects, common with cellular immunotherapies, can include flu-like symptoms such as fever, nausea and fatigue. Each patient's experience is different, but Malherbe's side effects were particularly challenging. He spiked a fever and his body ached. He experienced hallucinations and developed a full-body rash.
"I knew it was going to be tough, but it was much more than I expected," Malherbe said. "You have to be both physically and mentally prepared for this therapy."
Life is slowly getting back to normal for Malherbe, who is now 41. He returned to his job at an equine hospital earlier this month, and his scans show his lung nodules are shrinking, a sign the therapy is working.
"I am thankful for the great support I have received from my family, friends and coworkers over the past seven years. I am also grateful for the care team at Moffitt. I know I wasn't easy to deal with following my Tecelra infusion, but they are saints. I have so much respect for them and the kindness they have shown me and my family."
Malherbe’s eight-week post-treatment scans revealed his tumors had shrunk 50%, and he will continue to be monitored every few months. Although he is experiencing fatigue after strenuous activity, he feels great and got the go-ahead from his doctors to celebrate with his first glass of wine in months.