Revolutionizing Radiation Therapy for Soft Tissue Sarcomas
Soft tissue sarcomas are a group of rare cancers that arise in the connective tissues of the body, such as muscles, tendons and fat. Soft tissue sarcoma tumors vary greatly from one patient to another and even within different areas of the same tumor. Traditional treatments, including radiation therapy, do not often deliver the best possible outcomes.
Enter the habitat escalated adaptive therapy (HEAT), a phase 2 trial already deemed an early success in how soft tissue sarcomas are treated. The therapy’s goal is to personalize radiation treatment, making it more effective by adapting to the unique characteristics of each tumor. The trial’s findings were recently presented at the American Society for Radiation Oncology’s annual meeting.
“Soft tissue sarcomas make up less than 1% of all malignancies, and they can occur anywhere in the body from head to toe. It is a critical topic because of its rarity,” said Arash Naghavi, MD, the sarcoma section head in the Radiation Oncology Department at Moffitt Cancer Center, and the trial’s principal investigator. “Sarcoma is one of those few types of diseases where it becomes essential for us to use the technology we have to personalize care for the patient.”

Arash Naghavi, MD
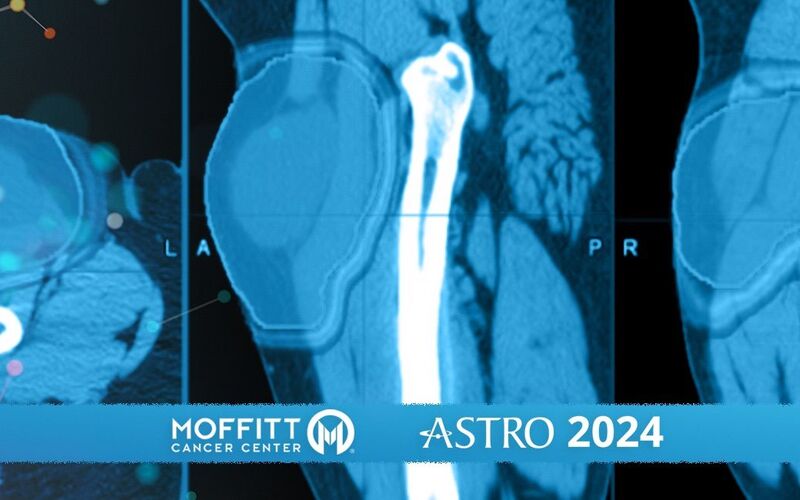
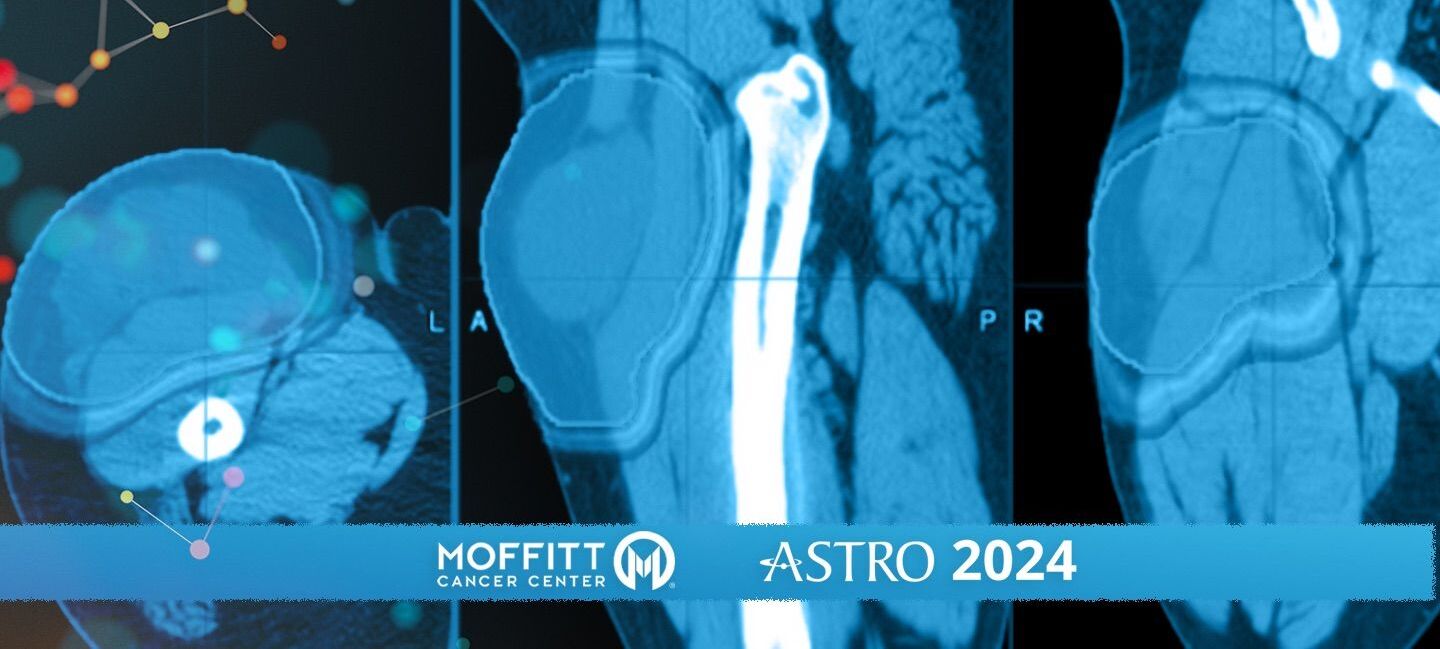
Traditionally, radiation therapy for soft tissue sarcomas has been a one-size-fits-all approach, with patients receiving similar doses regardless of the tumor’s specific characteristics. The HEAT trial seeks to change that by using a gene signature to determine the optimal radiation dose in sarcoma, known as the genomic adjusted radiation dose. With computer assisted analysis of a patient’s imaging, the HEAT trial determines the areas of the tumor that are the most aggressive and resistant to radiation and targets them with the optimal genomic dose. This allows for focal escalation of treatment for these aggressive radiomic habitats, while minimizing damage to the surrounding healthy tissue.
“If you can kill more of the cancer by the time the patient goes to surgery, they have improvement in local control, distant control and potentially even survival,” Naghavi explained. “In addition to that, the margins have a higher probability of being clear. Clear margins are important, and you can imagine if there's no cancer left, it's easy to get clear margins.”
The trial also monitors a patient’s response to treatment at the molecular level. Blood samples are collected to detect circulating tumor DNA, which can indicate whether the treatment is reducing the cancer’s spread.
“Patients will then get an MRI about two weeks into treatment. This MRI is to see what the biology is doing. We're able to stay one step ahead of the cancer by adjusting our radiation dose based off the response during treatment,” Naghavi said.
The trial included a patient group with an average age of 60 and a tumor size of 16.6 centimeters. It initially aimed to improve radiation response rates from a baseline of 8% to 24%. To be considered successful, seven of the first 23 patients needed to achieve this outcome. After 13 patients, eight reached the target, translating to a 62% response rate and exceeding the trial’s goals.
HEAT represents a major shift in how soft tissue sarcomas are treated, offering hope to patients that have historically had limited options. As the trial continues, researchers are gathering data that could pave the way for broader use of genomics and personalized radiation in cancer treatment.
“As we move forward, instead of thinking about these incremental changes, we really need to start looking into the biology and using the technology that we have to continue to make strides in pushing the field,” Naghavi said. “I think once we start to personalize care for patients, the sky's the limit because we would be able to do it for anyone.”