Brain Metastasis Treatment
Brain metastasis occurs when cancer cells from another part of the body spread to the brain. This serious complication of advanced-stage cancer begins when malignant cells detach from a primary tumor, travel through the bloodstream or lymphatic system and form new tumors in the brain. More common than primary brain tumors, brain metastases affect thousands of people each year, particularly those with cancers of the lung, breast, skin (melanoma), kidney or colon.
Depending on the location, size and number of tumors, brain metastasis can cause a wide range of neurological symptoms, which can appear suddenly or develop gradually. Some people experience persistent headaches, nausea or vomiting, while others have seizures, visual disturbances, speech difficulties, weakness on one side of the body or cognitive issues, such as memory loss or confusion.
The diagnostic process for brain metastasis typically begins with a neurological examination and imaging studies, such as magnetic resonance imaging (MRI) or computed tomography (CT) scans. In some cases, a biopsy or cerebrospinal fluid (CSF) analysis is needed to confirm the diagnosis and identify the origin of the primary cancer.
Brain metastasis treatment should be tailored to the patient’s unique medical needs after taking into account multiple factors, including the location, size and number of brain tumors, the type and stage of the primary cancer and the patient’s overall health and treatment goals. Options may include:
Surgery for brain metastasis treatment
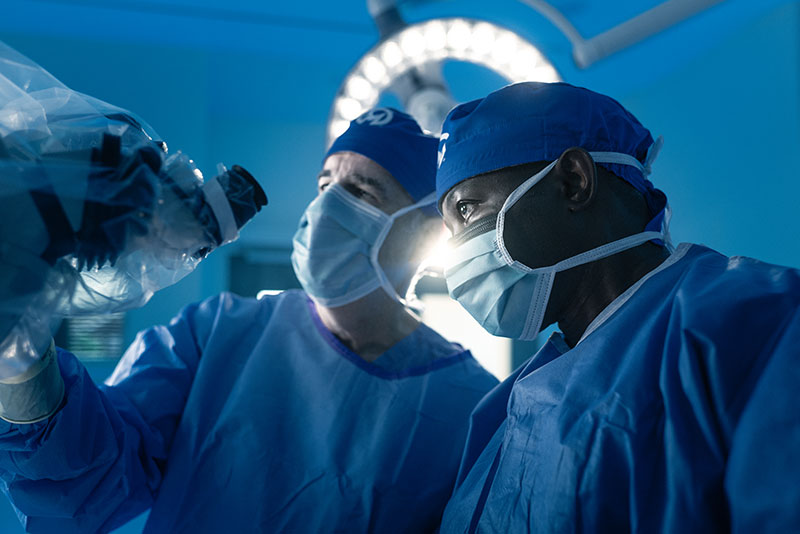
Surgical treatment for brain metastasis may be considered if the tumor is accessible, well-defined and causing significant neurological symptoms by creating intracranial pressure or swelling. The goal of surgery is to remove as much of the tumor as safely possible to relieve the symptoms and improve the patient’s quality of life. In many cases, the procedure is followed by radiation therapy or systemic therapy to target any remaining cancer cells.
Who is a candidate for brain metastasis surgery?
Brain metastasis surgery may be considered for a patient who has:
- A limited number of brain metastases (typically one or two)
- Tumors that are surgically accessible without affecting critical brain structures
- Good overall health and performance status
- Controlled or treatable primary cancer
What does brain metastasis surgery involve?
The most common procedure for brain metastasis is craniotomy with tumor resection, which involves temporarily removing a portion of the skull to gain direct access to the tumor. The surgeon will then carefully excise the tumor while minimizing any impact on the surrounding healthy brain tissues.
What are the potential benefits of brain metastasis surgery?
In certain cases, brain metastasis surgery can allow for:
- Immediate relief from symptoms caused by mass effect, such as headaches, seizures and neurological deficits
- Tissue sample collection for biopsy and diagnostic purposes
- A good outcome and quality of life
What are the risks and possible complications of brain metastasis surgery?
Like any surgical procedure, brain metastasis surgery carries certain risks, including:
- Infection at the surgical site
- Excessive bleeding
- Intracranial swelling
- Neurological deficits, which can vary depending on the tumor location
What is the recovery process like?
Recovery from brain metastasis surgery can vary widely depending on the complexity of the procedure and the characteristics of the patient. Postoperative imaging is typically performed to assess the success of the tumor removal, and many patients begin supplemental therapies soon afterward.
 Florida's Largest NCI Cancer Center
Florida's Largest NCI Cancer Center
Recognized as a leader in cancer excellence, Moffitt is the state's largest National Cancer Institute-designated Comprehensive Cancer Center by cancer patient volumes.
Radiation therapy for brain metastasis
Radiation therapy is a key treatment for brain metastasis, using high-energy beams to destroy cancer cells or inhibit their growth. It may be used alone or in combination with surgery or systemic treatment, depending on the patient’s needs. The specific approach is guided by several factors, including the location, size and number of tumors, the type of primary cancer and the patient’s overall health. This targeted treatment can help relieve symptoms, improve the outcome and enhance the patient’s quality of life.
Several forms of radiation therapy may be used to treat brain metastasis. These include:
Stereotactic radiosurgery (SRS) for brain metastasis
SRS is a noninvasive, targeted treatment that can accurately deliver a high dose of radiation to one or a few small tumors in the brain. Despite its name, stereotactic radiosurgery does not involve a surgical incision. This advanced form of radiation therapy is:
- Typically completed in one to five sessions
- Generally ideal for a patient with a limited number of brain metastases
- Highly precise, allowing for the preservation of healthy brain tissues by targeting only the tumors
- Often used after surgery to treat residual cancer or as a primary treatment for an inoperable tumor
Whole-brain radiation therapy (WBRT) for brain metastasis
WBRT may be considered to address multiple or “seeded” brain metastases, which have spread throughout the brain tissues. By treating the entire brain with high-dose radiation, whole-brain radiation therapy can:
- Help control widespread disease
- Alleviate neurological symptoms, such as headaches, seizures and mental confusion
- Be used for supportive care when curative treatment is not possible
Fractionated stereotactic radiotherapy (FSRT)
FSRT delivers precise, low doses of radiation over multiple sessions, which may be well-suited for addressing a large tumor or a lesion positioned near an especially delicate area of the brain. This approach can minimize damage to surrounding healthy tissues while maintaining treatment effectiveness.
What are the potential benefits of radiation therapy for brain metastasis?
Radiation therapy for brain metastasis is a noninvasive treatment that is well-tolerated by many patients. By slowing or halting cancer growth, it can potentially shrink a brain tumor and relieve the related neurological symptoms, such as headaches, weakness and seizures. It can also be repeated in some cases if new metastases develop.
What are the possible side effects of radiation therapy for brain metastasis?
The side effects of radiation therapy can vary depending on the delivery method and the patient’s response. Some patients temporarily experience:
- Fatigue
- Hair loss in the treatment area
- Headaches
- Memory changes or cognitive difficulties (more common with WBRT)
- Swelling or inflammation in the brain (may require steroid treatment)


One of the World's Best Cancer Hospitals
Schedule an AppointmentTargeted therapy for brain metastasis
Targeted therapy is a precision-based cancer treatment that uses specialized medications to interfere with specific genetic mutations or molecular changes responsible for tumor growth. Unlike traditional chemotherapy, which affects all rapidly dividing cells—including healthy cells—targeted therapy is designed to act specifically on cancer cells. This focused approach may reduce side effects and improve outcomes for some patients. In cases of brain metastasis, targeted therapy can be particularly effective when the primary cancer has identifiable mutations or biomarkers that are responsive to these drugs.
How does targeted therapy for brain metastasis work?
Based on the results of comprehensive molecular profiling, targeted therapy drugs can be developed to:
- Block signals that stimulate cancer cells to grow and divide
- Inhibit the formation of new blood vessels that supply nutrients to tumors (anti-angiogenesis)
- Induce cancer cell death by interfering with key proteins or enzymes involved in cell survival
- Penetrate the blood-brain barrier in some cases, enabling more effective treatment of brain tumors
What primary cancers may respond to targeted therapy?
Targeted therapy is most effective for treating brain metastasis that stems from a primary tumor with identifiable, actionable genetic mutations, such as:
- Non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), ROS1 or other gene mutations, rearrangements or fusions
- Breast cancer with human epidermal growth factor receptor 2 (HER2)-positive tumors
- Melanoma with BRAF gene mutations
- Colorectal cancer with certain HER2 or KRAS gene mutations
Molecular testing of the primary tumor or brain metastasis is essential to determine whether the patient is eligible for targeted therapy.
What drugs can be used for targeted therapy?
Depending on the primary cancer type and its genetic profile, the following targeted drugs may be considered for brain metastasis:
- EGFR inhibitors – Osimertinib for EGFR-mutated lung cancer
- ALK inhibitors – Alectinib or lorlatinib for ALK-positive lung cancer
- HER2 inhibitors – Tucatinib or neratinib for HER2-positive breast cancer
- BRAF inhibitors – Dabrafenib combined with trametinib for BRAF-mutant melanoma
What are the potential benefits of targeted therapy for brain metastasis?
In select cases, targeted therapy for brain metastasis can:
- Provide a noninvasive treatment option, often administered as a pill or intravenous (IV) infusion
- Help shrink or stabilize brain tumors
- Delay or reduce the need for radiation therapy or surgery
- Result in fewer systemic side effects than traditional chemotherapy
What are the possible side effects of targeted therapy for brain metastasis?
While generally better tolerated than traditional chemotherapy, targeted therapy can still cause side effects, such as:
- Fatigue
- Skin rash
- Diarrhea
- Elevated liver enzymes
- Risk of resistance over time as cancer cells adapt
Immunotherapy for brain metastasis
Immunotherapy is an emerging treatment option for some patients with brain metastasis. It aims to enhance the body’s natural immune response, helping immune cells recognize and destroy cancer cells more effectively. Unlike traditional therapies that act directly on the tumor, immunotherapy activates the immune system to target cancerous tissues, including tumors within the brain. This approach can be especially valuable given the challenges of treating brain tumors due to the protective nature of the blood-brain barrier.
What does immunotherapy for brain metastasis involve?
The most common form of immunotherapy used to treat brain metastasis is immune checkpoint inhibitor therapy. This approach works by blocking the signals that some cancer cells use to suppress the immune response, effectively releasing the “brakes” and allowing immune cells to attack the tumor. These medications are typically prescribed for cancers that exhibit specific traits, such as a high tumor mutation burden or the presence of particular protein markers like PD-L1.
What primary cancers may respond to immunotherapy?
Immunotherapy may be considered to address brain metastasis originating from:
- Melanoma
- Non-small cell lung cancer
- Renal cell carcinoma (kidney cancer)
- Triple-negative breast cancer
What immunotherapy drugs can be used for brain metastasis?
Several checkpoint inhibitors have been approved or are currently in clinical use for treating brain metastasis. These include:
- PD-1 inhibitors – Pembrolizumab and nivolumab
- PD-L1 inhibitors – Atezolizumab and durvalumab
- CTLA-4 inhibitors – Ipilimumab
In some cases, checkpoint inhibitors may be combined for enhanced effectiveness.
What are the potential benefits of immunotherapy for brain metastasis?
As a noninvasive treatment for brain metastasis, immunotherapy can:
- Be administered through IV infusion on an outpatient basis
- Shrink or stabilize brain tumors in certain patients
- Be combined with other treatments, such as radiation therapy, surgery or targeted therapy, for greater effectiveness
- Improve the outcome and enhance the patient’s quality of life
Additionally, some immunotherapy agents have shown the ability to cross the blood-brain barrier or modulate immune activity within the central nervous system, making them a promising option for treating tumors in the brain.
What are the possible side effects of immunotherapy for brain metastasis?
Immunotherapy can potentially trigger a variety of immune-related side effects, primarily because the activated immune system may also attack healthy tissues. Some patients may temporarily experience:
- Fatigue
- Skin rash or itching
- Diarrhea or colitis
- Inflammation of the lungs (pneumonitis)
- Hormone-related issues (e.g., thyroid or adrenal gland dysfunction)
These side effects should be closely monitored and are typically manageable with supportive care or corticosteroids.
Intrathecal therapy for brain metastasis
Intrathecal therapy is a specialized method of delivering medication directly into the cerebrospinal fluid. This approach is primarily used to treat cancers that have spread to the central nervous system, such as leptomeningeal metastasis, which occurs when cancer cells infiltrate the thin protective membranes (leptomeninges) surrounding the brain and spinal cord.
Unlike traditional chemotherapy, which is administered through the bloodstream and often limited by the blood-brain barrier, intrathecal therapy delivers medication precisely where it is needed. For certain patients with brain metastasis, this targeted approach offers a more direct—and potentially more effective—treatment option.
How does intrathecal therapy for brain metastasis work?
Unlike conventional chemotherapy, which circulates through the bloodstream, intrathecal therapy delivers powerful anticancer drugs directly into the cerebrospinal fluid, allowing the medication to more effectively reach cancer cells within the brain and spinal cord. Intrathecal therapy is typically administered in one of two ways:
- Lumbar puncture (spinal tap) – A needle is inserted into the lower back to inject the medication directly into the CSF.
- Ommaya reservoir – A small, dome-shaped device is surgically placed under the scalp and connected to a catheter in the brain’s ventricles. This system enables repeated access to the CSF without the need for multiple spinal punctures.
Both methods help ensure that high concentrations of chemotherapy reach the central nervous system, an area that systemic treatments often have difficulty penetrating effectively.
When is intrathecal therapy used for brain metastasis?
Intrathecal therapy is not suitable for all cases of brain metastasis. It may be considered for a patient with:
- Leptomeningeal metastasis
- Evidence of malignant cells in the cerebrospinal fluid
- Neurological symptoms linked to cancer affecting the meninges
- Limited or no response to traditional systemic therapies
This targeted approach is typically considered only after other treatments have proven ineffective or when cancer has specifically spread to the central nervous system.
What drugs can be used for intrathecal therapy for brain metastasis?
Several chemotherapeutic agents may be administered intrathecally, depending on the patient’s cancer type and clinical status. These include:
- Methotrexate – One of the most widely used drugs for intrathecal administration
- Cytarabine – Often used in a slow-release (liposomal) formulation for prolonged effect
- Thiotepa – Occasionally used for specific cancers
These medications may be administered alone or in combination, depending on the patient’s tolerance and treatment goals.
What are the potential benefits of intrathecal therapy for brain metastasis?
When used for brain metastasis, intrathecal therapy can potentially:
- Deliver medication directly into the cerebrospinal fluid and affected areas of the central nervous system
- Reduce or stabilize neurological symptoms caused by leptomeningeal metastasis
- Improve the outcome and quality of life for select patients when used in combination with other therapies
What are the possible side effects of intrathecal therapy for brain metastasis?
Like all cancer treatments, intrathecal therapy carries a risk of side effects, which can vary depending on the drug used and other factors. Some patients temporarily experience:
- Headaches
- Nausea or vomiting
- Fatigue
- Localized pain or discomfort
- Increased risk of infection (particularly with an implanted device)
- Neurological complications, such as confusion or seizures (rare)
Close monitoring and supportive care can be provided to manage these side effects and help ensure a comfortable experience for the patient.
Benefit from world-class care at Moffitt Cancer Center
If you would like to learn more about brain metastasis treatment, you can request an appointment with a specialist in the Neuro-Oncology Program at Moffitt by calling 1-888-663-3488 or submitting a new patient registration form online. We do not require referrals.
Brain Metastases
